Clinical Trials
Department of Clinical Trials at CARe Keralam offers full drug development services. We specialize in strategic development, management, execution and analysis of all Phase II to Phase IV clinical studies of herbal drugs and other alternative medicines. This department is all set to efficiently, ethically scientifically execute clinical research programs to promote better medical care for the whole mankind.
The mission of this department is to help herbal companies and research institutions world-wide in conducting high quality clinical research trials globally with an emphasis on reducing clinical trial timelines and cost by applying deep therapeutic expertise. We give utmost importance to patient rights, safety and also ensureall required confidentialities
Services Offered
Preclinical studies ( toxicology, animal efficacy studies)
Clinical Trial Budgeting
Biostatistics service
- Preparation of Study Design
- Determination of sample size based on power analysis
- Interim Analysis
- Final Analysis & Report
Medical writing
- Preparation of Protocol
- Investigator Brochure (IB) or Product Dossier
- Case Report Forms (CRFs)
- Informed consent form (ICF)
- Subject Diaries
- Study logs (delegation log, IP logs etc.)
Regulatory submissions
- DCGI Submissions
- EC submissions
- CTRI (Clinical Trials Registry- India) Registration
- ClinicalTrials.gov Registration
Operations
- Site selection& Site management
- Trial Management
- IP and Trial Material Management
- Data Management
- Monitoring
- Clinical Study Report
Documentation
- Documentation of clinical trial ( TMF,PMF, SMF )
- Quality System Management
- Case Report Forms (CRFs)
- SOP Development
What Is a Clinical Study?
A clinical study involves research using human volunteers (also called participants) that is intended to add to medical knowledge. There are two main types of clinical studies: clinical trials and observational studies. ClinicalTrials.gov includes both interventional and observational studies.
Why Clinical study?
Its a law GSR 377(E) of drugs and cosmetics Rule 1945 which tells that , At present clinical trial is not required for classical or generic ASU drugs but Patent or proprietary medicine require evidence of effectiveness vide GSR 377(E) of drugs and cosmetics Rule 1945.
Clinical trials will help to confirm whether a new approach works well in people and is safe and effective. They may also be done to find treatments with fewer side effects, or treatments that are easier for patients to tolerate
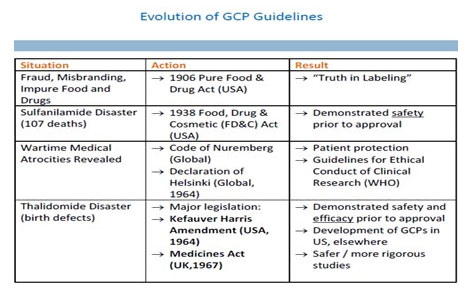
Evolution of Regulations.
The evolution of the regulatory process have allowed for a significant improvement in patient care, safety, and clinical outcomes. Undoubtedly, medicine will continue to mature and adapt to new advances and ever-changing world politics to the overall betterment of clinical research and, ultimately, the well-being of the world population. The regulations are intended to protect the rights, safety and well-being of research participants and to simplify and harmonise regulatory processes. They apply to trials designed to generate information on the efficacy or safety of medicines.
Good Clinical Practice (GCP)
GCP is an international quality standard that is provided by International Conference on Harmonisation (ICH), an international body that defines standards, which governments can transpose into regulations for clinical trials involving human subjects. Which includes standards on how clinical trials should be conducted, define the roles and responsibilities of clinical trial sponsors, clinical research investigators, and monitors. In the pharmaceutical industry monitors are often called Clinical Research Associates.
MARCH 2013 Department of AYUSH, Ministry of Health & Family Welfare, Government of India introduced AYUSH GCP guidelines (GCP-ASU)
Clinical Trials
In a clinical trial (also called an interventional study), participants receive specific interventions according to the research plan or protocol created by the investigators. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants' behavior, for example, diet. Clinical trials may compare a new medical approach to a standard one that is already available or to a placebo that contains no active ingredients or to no intervention. Some clinical trials compare interventions that are already available to each other. When a new product or approach is being studied, it is not usually known whether it will be helpful, harmful, or no different than available alternatives (including no intervention). The investigators try to determine the safety and efficacy of the intervention by measuring certain outcomes in the participants. For example, investigators may give a drug or treatment to participants who have high blood pressure to see whether their blood pressure decreases.
Note: Some people who are not eligible to participate in a clinical trial may be able to get experimental drugs or devices outside of a clinical trial through an Expanded Access Program. See more information on expanded access from the National Library of Medicine.
Observational Studies
In an observational study, investigators assess health outcomes in groups of participants according to a protocol or research plan. Participants may receive interventions, which can include medical products, such as drugs or devices, or procedures as part of their routine medical care, but participants are not assigned to specific interventions by the investigator (as in a clinical trial). For example, investigators may observe a group of older adults to learn more about the effects of different lifestyles on cardiac health.
Who Conducts Clinical Studies?
Every clinical study is led by a principal investigator, who is often a medical doctor. Clinical studies also have a research team that may include doctors, nurses, social workers, and other health care professionals.
Clinical studies can be sponsored, or funded, by pharmaceutical companies, academic medical centers, voluntary groups, and other organizations, in addition to Federal agencies such as the National Institutes of Health, U.S. Department of Defense, and U.S. Department of Veterans Affairs. Physicians, health care providers, and other
Where Are Clinical Studies Conducted?
Clinical studies can take place in many locations, including hospitals, universities, doctors' offices, and community clinics. The location depends on who is conducting the study.
How Long Do Clinical Studies Last?
The length of a clinical study varies, depending on what is being studied. Participants are told how long the study will last before enrolling.
Reasons for Conducting Clinical Studies
In general, clinical studies are designed to add to medical knowledge related to the treatment, diagnosis, and prevention of diseases or conditions. Some common reasons for conducting clinical studies include:
- Evaluating one or more interventions (for example, drugs, medical devices, approaches to surgery or radiation therapy) for treating a disease, syndrome, or condition
- Finding ways to prevent the initial development or recurrence of a disease or condition. These can include medicines, vaccines, or lifestyle changes, among other approaches.
- Evaluating one or more interventions aimed at identifying or diagnosing a particular disease or condition Examining methods for identifying a condition or risk factors for that condition
- Exploring and measuring ways to improve the comfort and quality of life of people with a chronic illness through supportive care
Participating in Clinical Studies
A clinical study is conducted according to a research plan known as the protocol. The protocol is designed to answer specific research questions as well as safeguard the health of participants. It contains the following information:
- The reason for conducting the study
- Who may participate in the study (the eligibility criteria)
- The number of participants needed
- The schedule of tests, procedures, or drugs and their dosages
- The length of the study
- What information will be gathered about the participants
- Who Can Participate in a Clinical Study?
Clinical studies have standards outlining who can participate, called eligibility criteria, which are listed in the protocol. Some research studies seek participants who have the illnesses or conditions that will be studied. Other studies are looking for healthy participants. And some studies are limited to a predetermined group of people who are asked by researchers to enroll.
Eligibility: The factors that allow someone to participate in a clinical study are called inclusion criteria, and the factors that disqualify someone from participating are called exclusion criteria. These are based on things such as age, gender, the type and stage of a disease, previous treatment history, and other medical conditions.
How Are Participants Protected?
Informed consent is a process in which researchers provide potential and enrolled participants with information about a clinical study. This information helps people decide whether they want to enroll, or continue to participate, in the study. The informed consent process is intended to protect participants and should provide enough information for a person to understand the risks of, potential benefits of, and alternatives to the study. In addition to the informed consent document, the process may involve recruitment materials, verbal instructions, question-and-answer sessions, and activities to measure participant understanding. In general, a person must sign an informed consent document before entering a study to show that he or she was given information on risks, potential benefits, and alternatives and understands it. Signing the document and providing consent is not a contract. Participants may withdraw from a study at any time, even if the study is not over. See Questions to Ask a health care provider or researcher about participating in a clinical study.
Institutional review boards:
Each federally supported or conducted clinical study and each study of a drug, biological product, or medical device regulated by FDA must be reviewed, approved, and monitored by an institutional review board (IRB). An IRB is made up of physicians, researchers, and members of the community. Its role is to make sure that the study is ethical and the rights and welfare of participants are protected. This includes making sure that research risks are minimized and are reasonable in relation to any potential benefits, among other things. The IRB also reviews the informed consent document.
In addition to being monitored by an IRB, some clinical studies are also monitored by data monitoring committees (also called data safety and monitoring boards).
Various Federal agencies, including the Office of Human Subjects Research Protection (OHRP) and FDA, have the authority to determine whether sponsors of certain clinical studies are adequately protecting research participants.
Relationship to Usual Health Care
Typically participants continue to see their usual health care providers while enrolled in a clinical study. While most clinical studies provide participants with medical products or interventions related to the illness or condition being studied, they do not provide extended or complete health care. By having the participant's usual health care provider work with the research team, the participant can make sure that the study protocol will not conflict with other medications or treatments being received.
Considerations for Participation
Participating in a clinical study contributes to medical knowledge. The results of these studies can make a difference in the care of future patients by providing information about the benefits and risks of therapeutic, preventative, or diagnostic products or interventions.
Clinical trials provide the basis for the development and marketing of new drugs, biological products, and medical devices. Sometimes, the safety and the effectiveness of the experimental approach or use may not be fully known at the time of the trial. Some trials may provide participants with the prospect of receiving direct medical benefits, while others do not. Most trials involve some risk of harm or injury to the participant, although it may not be more than the risks related to routine medical care or disease progression. (For trials approved by IRBs, the IRB has decided that the risks of participation have been minimized and are reasonable in relation to anticipated benefits.) Many trials require participants to undergo additional procedures, tests, and assessments based on the study protocol. These will be described in the informed consent document for a particular trial. A potential participant should also discuss these issues with members of the research team and with his or her usual health care provider.
Clinical research organization or Contract Research Organization
A contract research organization (CRO) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis.
Send your queries regarding Clinical Trials.
Mob: +91 8129688999
